
Class 12 Chemistry Chapter -3 Chemical Kinetics Notes [English Medium]Updated – MyNoteswala
Highlight of the chapter.
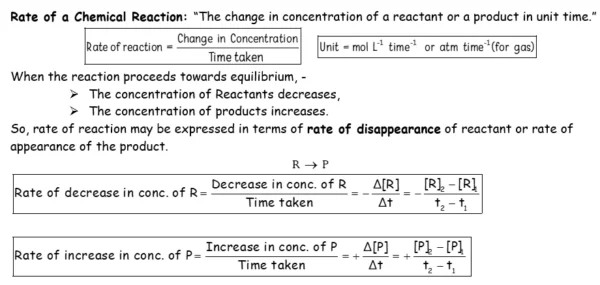
- Rate of reaction and its unit.
- Define the average and instantaneous rate of a reaction;
- Express the rate of a reaction in terms of change in concentration of either of the reactants or products with time.
- Distinguish between elementary and complex reactions.
- Differentiate between the molecularity and order of a reaction.
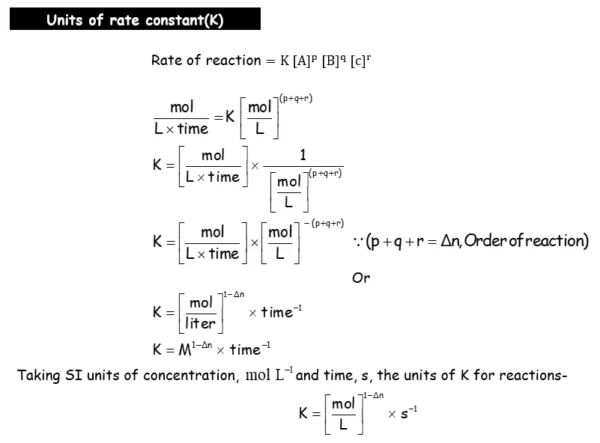
- Define rate constant (K).
- Discuss the dependence of rate of reactions on concentration, temperature and catalyst.
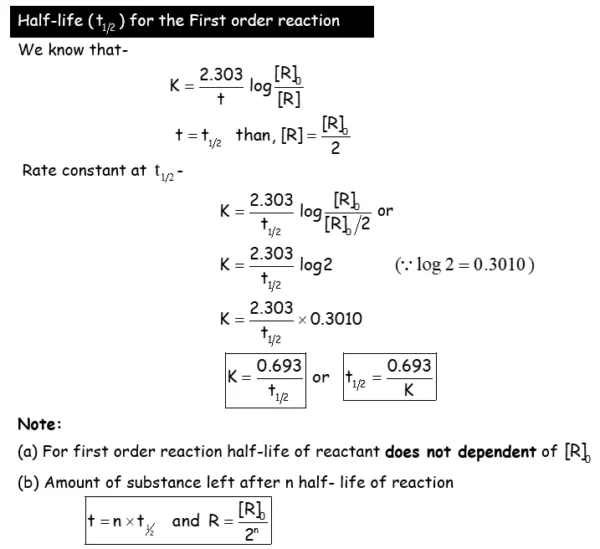
- Derive integrated rate equations for the zero and first order reactions.
- Determine the rate constants for zeroth and first order reactions.
- Describe collision theory and Arrhenius equation.









![Class 12 Chemistry Chapter 1 to 10 [English Medium] – MyNoteswala](https://mynoteswala.com/wp-content/uploads/2023/08/Class-12-Chemistry-all-chapters-English-medium-300x300.webp)
![Class 12 Chemistry Chapter 2 Electrochemistry [English Medium] PDF – MyNoteswala](https://mynoteswala.com/wp-content/uploads/2024/02/Unit-2-Electrochemistry-300x300.png)
![Class 12 Chemistry Chapter 4 d- and f- Block Elements [English Medium] – MyNoteswala](https://mynoteswala.com/wp-content/uploads/2023/07/Class-12-Chemistry-chapter-4-English-Medium-300x300.webp)
![Class 12 Chemistry Chapter 1 to 10 [English Medium] PDF – MyNoteswala](https://mynoteswala.com/wp-content/uploads/2024/05/Class-12-Unit-1-to-10-English-Medium--300x300.png)
![Class 12 Chemistry Chapter 7 Alcohol, Phenol and Ether [English Medium] PDF – MyNoteswala](https://mynoteswala.com/wp-content/uploads/2024/01/Unit-7-Alcohol-phenol-and-Ether-300x300.png)
![Class 12 Chemistry Chapter 5 Coordination Chemistry [English Medium] PDF – MyNoteswala](https://mynoteswala.com/wp-content/uploads/2024/02/Unit-5-Coordination-Chemistry-300x300.png)
![Class 12 Chemistry Chapter 3 Chemical Kinetics [English Medium] – MyNoteswala](https://mynoteswala.com/wp-content/uploads/2023/07/Class-12-Chemistry-chapter-3-English-Medium-300x300.webp)
Reviews
There are no reviews yet.