
Class 12 Chemistry Chapter -2 Electrochemistry Notes [English Medium]Updated – MyNoteswala
Highlight of the chapter.
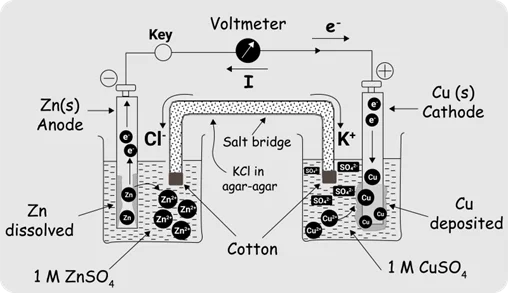
- Describe an electrochemical cell and differentiate between galvanic and electrolytic cells.
- Standard H- electrode (SHE).
- Electrochemical series.
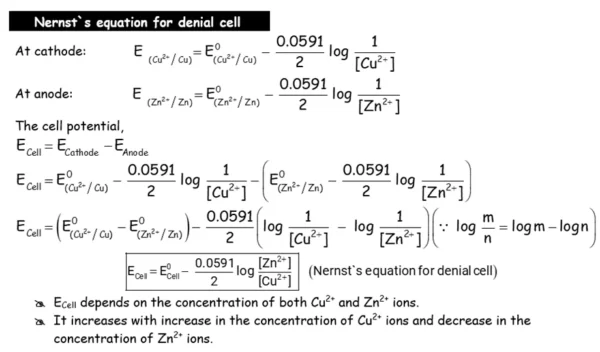
- Apply Nernst equation for calculating the emf of galvanic cell and define standard potential of the cell.
- Derive relation between standard potential of the cell, Gibbs energy of cell reaction and its equilibrium
constant. - Define resistivity , conductivity and molar conductivity (λm) of ionic solutions.
- Differentiate between ionic (electrolytic) and electronic conductivity;
- Measurement of conductivity of electrolytic solutions and calculation of their molar conductivity;
- Explanation of variation of conductivity and molar conductivity of solutions with change in their concentration and define molar conductivity at zero concentration or infinite dilution).
- Kohlrausch law and its applications.
- Describe the construction of some primary and secondary batteries and fuel cells.
- Explain corrosion as an electrochemical process (Rusting of iron).
















Reviews
There are no reviews yet.